Which Statement Best Describes Hydrogen Bonding
It is formed only between different molecules intermolecular. It is a strong chemical bond formed by the sharing of valence electrons.

Hydrogen Bonding Chemistry For Non Majors
3 The electronegative hydrogen atom in one.

. Electrostatic attractions between pairs of electrons and positively charged nuclei. Only van der Waals forces. Why is it D not C.
Structure of Carbon Therefore carbon atoms can form four covalent bonds with other atoms to. 9 Why do water molecules stick to other water molecules quizlet. 2 The electropositive hydrogen atom in one molecule and an electropositive atom in another molecule experience attraction.
Which of the following best describes ionic bonding. The transfer of valence electrons between atoms to make them neutral. 452 ev is exposed to an ultraviolet light source.
The true statement about the hydrogen bond is c. Which statement best explains why a carbon atom can bond with itself easily It is an ion It has four valence electrons it is an organic element it forms hydrogen bonds. The sharing of valence electrons between two or more neutral atoms c.
What You Mean By Hydration. What changes will be observed if. 200 nm which results in the ejection of photoelectrons.
10 How do water molecules interact. Which of the following best characterizes a hydrogen bond. H Cl HCl.
Electrostatic attractions between H and CN ions. 11 Which statement is true about water molecules. School Western Philippines University.
7 Why do hydrogen bonds dissolve in water. The repulsion of ions due to the transfer of valence electrons d. Which statement best describes hydrogen bonding.
35 _____ A Weak interactions that become significant when molecules are very close together B Very strong bonding between cations and anions C Electrostatic attraction between charged areas of adjacent molecules D Very strong bonding from the sharing of electrons between atoms. There are 2 direct hydrogen bonds between the ligand and hemoglobin amino acids. Which statement best describes hydrogen bonding.
There are no hydrogen bonds between the ligand and hemoglobin amino acids. Which statement best describes hydrogen bonding. 2In both structures the most abundant noncovalent interactions are the multiple hydrophobic interactions between the.
The electronegative hydrogen atom in one molecule and an. 1 The electronegative hydrogen atom in one molecule and an electropositive atom in another molecule experience attraction. In order to sell your land you need to.
Which statement best describes hydrogen bonding. Which statement best describes a hydrogen bond a It a special type of dipole. 1Which of the following statements best describes the hydrogen bonding network between deoxyhemoglobin 2HHB and the.
Which statement best describes hydrogen bonding. This can be seen when hydrogen atom combine with chlorine atom to form hydrogen chloride as shown below. A hydrogen bond is when a polar hydrogen containing molecule interacts with another polar molecule to make a weak non-covalent bond c.
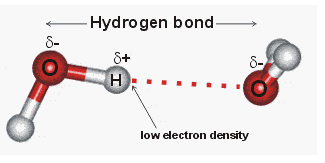
A hydrogen bond is the electromagnetic attraction created between a partially positively charged hydrogen atom attached to a highly electronegative atom and another. 35 Which of the following best describes hydrogen bonding. The true statement about the hydrogen bond is c.
T is a weak and temporary electrical attraction between two polar molecules. A true statement about the hydrogen bond is that it lies between C and O. The correct answer.
There are no direct hydrogen bonds between the ligand. Which statement best describes a hydrogen bond a it a. There are 2 direct hydrogen bonds between the ligand and hemoglobin amino acids.
Hydrogen bond is weak force between atoms in a molecule but is of enormous Which is true about the hydrogen bond. 12 How does hydrogen bonding between water molecules affect the properties of water. The attraction of ions due to the transfer of valence electrons b.
2 Get Other questions on the subject. The electronegative hydrogen atom in one molecule and an electropositive atom in another molecule experience attraction. Imagine that you own a property that is exactly 22 acres large.
96Which statement best describes the intramolecular bonding in HCN l. 3Which of the following statements is consistent with. Chemistry 22062019 0130 josephaciaful.
The correct answer. A hydrogen bond is when hydrogen shares electrons with another element creating a new molecule through a covalent bond. Bonding atoms in molecules has very weak force but it exerts enormous amounts of energy.
In this bonding electrons are shared between the two atoms involved in order to attain a stable octet configuration. Another question on Chemistry. Silver is replaced.
Which of the following statements best describes the hydrogen bonding network between deoxyhemoglobin 2HHB and the Fe-heme ligandThere are 2 direct hydrogen bonds between the ligand and hemoglobin amino acidsThere are no hydrogen bonds between the ligand and hemoglobin amino acidsThere are no direct hydrogen bonds between the ligand. Covalent bonding is a type of bonding which exist between two non metals. It forms only between nonpolar molecules.
Van der Waals forces and hydrogen bonding. Hydrogen bond is weak force between atoms in a molecule but is of enormous Related This entry was posted in Answers and tagged bonding hydrogen statement true. Question Which of the following statements best describes the hydrogen bonding network between deoxyhemoglobin 2HHB and the Fe-heme ligand.
There are no hydrogen bonds between the ligand and hemoglobin amino acids. Pages 21 This preview shows page 16 - 21 out of 21 pages. You want to sell your property but your realtor tells you that you cannot sell your land by the acre.
Asample of silver with work function. 2 Show answers Another question on Chemistry. 8 What best describes the bonding in water molecule.
A hydrogen bond is when a polar hydrogen containing molecule interacts with another polar molecule to. Course Title CHEM 2. From Something that takes up or mixes with water or another component of water.
Which of the following statements best describes the hydrogen bonding network between deoxyhemoglobin 2HHB and the Fe-heme ligand. Follow the steps provided in the simulation to add water to the graduated cylinder select one of the three samples copper silver or gold set its mass to the values given in the statements. The electropositive hydrogen atom in one molecule and an electropositive atom in another molecule experience attraction.

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

Hydrogen Bonding Definition Examples Facts Britannica

Solved Question 6 Which Of The Following Best Describes Chegg Com

Hydrogen Bonds In Water Article Khan Academy

Hydrogen Bond Ck 12 Foundation

The Two Hydrogen Bond Interaction Between Adenine A And Thymine T Download Scientific Diagram

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules
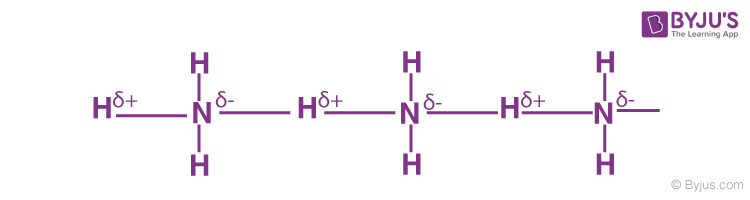
Hydrogen Bonding Occurs In Which Type Of Crystalline Solids Q A
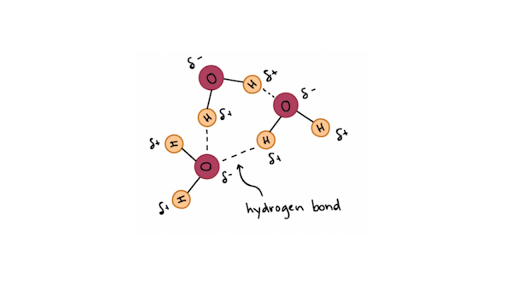
Hydrogen Bonds In Water Article Khan Academy

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules

Pin By Kkent On Time And Time Again Zodiac Signs Horoscope Libra Zodiac Facts Gemini Zodiac

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey
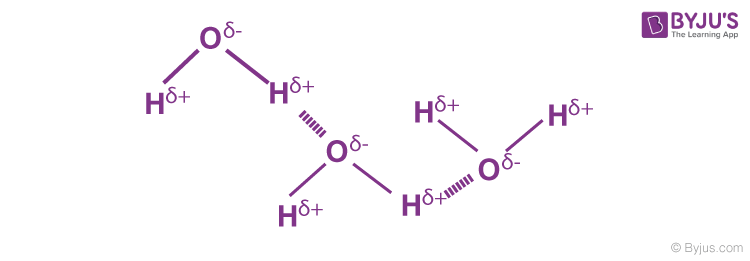
Hydrogen Bond Is Represented By A Byju S Q A

2 1j Hydrogen Bonding And Van Der Waals Forces Biology Libretexts

Top Hydrogen Bonding Capabilities Of The Theobromine And Caffeine Download Scientific Diagram
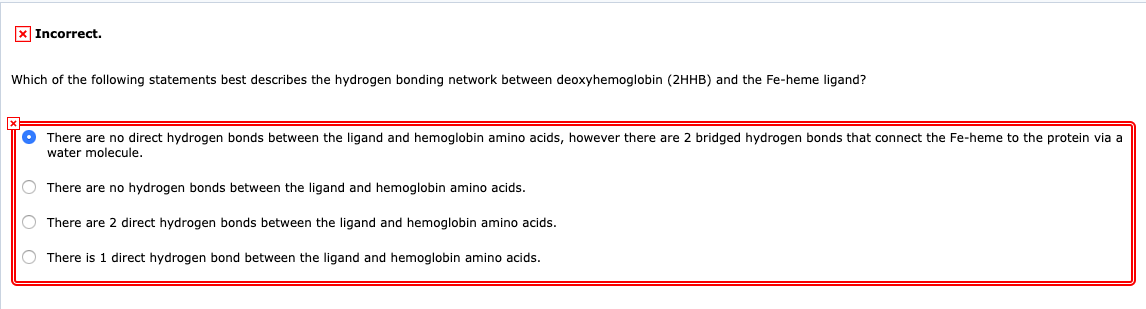
Solved X Incorrect Which Of The Following Statements Best Chegg Com
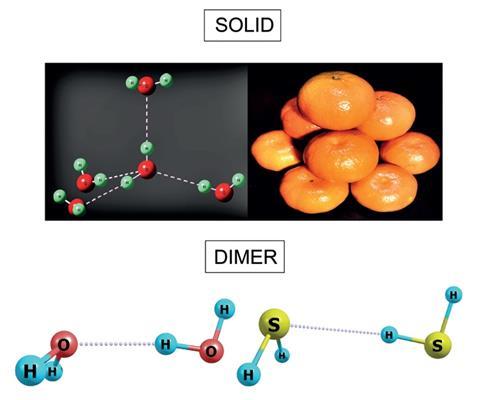
Hydrogen Sulfide Surprises As It S Discovered To Have Hydrogen Bonds Research Chemistry World
Comments
Post a Comment